Childhood International Protocol – Acute Myeloid Leukaemia 2022
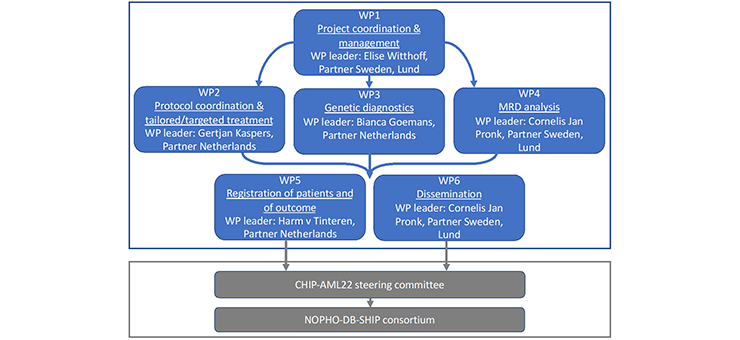
Background and goals to the CHIP-AML22 and the EU4Health programme grant
Central in oncology is the notion that the right treatment is offered to the right patients. This holds true also for paediatric acute myeloid leukemia (AML) where we today walk the fine balance between treatment efficacy versus treatment-related severe toxicity. The latter involves both morbidities as well as mortality. This severity in treatment is for instance illustrated by the fact that already after the first (of five) chemotherapy course for children with AML treated within our consortium, close to 100% are admitted to the hospital due to neutropenic fever with the risk of severe systemic infections.
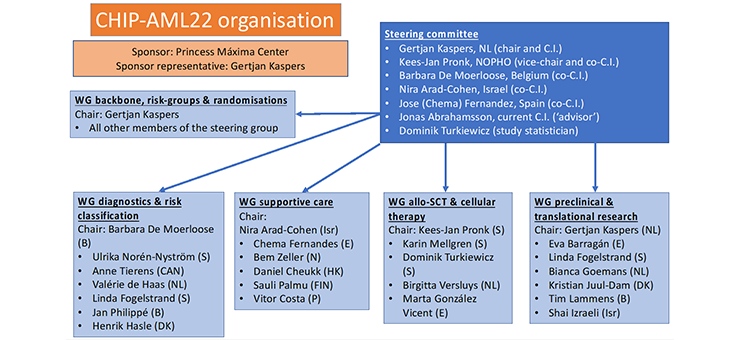
Genetic diagnostics have become central in modern AML treatment and will also be implemented in the CHIP-AML22 (Childhood International Protocol – AML2022) within our NOPHO-DB-SHIP consortium. The previous treatment protocol within our consortium, the NOPHO-DBH AML12 protocol (in short AML12), has taught us some valuable lessons; advanced frontline diagnostics, both genetic diagnostics of leukemic cells and treatment-response evaluation, will contribute to improved outcome for these patients. However, availability of such techniques, as well as availability of more modern targeted treatment (often targeting the genetic basis of this disease) is neither equally distributed among European countries in large, nor within our consortium. We are convinced that implementation of these frontline diagnostic and treatment possibilities in modern AML treatment within our consortium, and potentially among a much wider cohort of patients (including adult AML), will result in further improvement of overall outcome.
Objectives in the CHIP-AML22 and the EU4H grant
To reach the aforementioned goals, we focus on 3 important areas in the CHIP-AML22 study, that also have formed the basis of the EU4H application.
These overall objectives are:
- Genetic profiling of the leukemic cells, in combination with survival data, has allowed us to identify subgroups of patients with prognostic favourable and unfavourable genetic aberrations in the context of the AML12 study. Intensified treatment for the group with “unfavourable genetics” is necessary to avoid relapse, albeit that this intensification involves hematopoietic stem cell transplantation (SCT) which largely adds to treatment toxicity. Advances in next generation sequencing (NGS, such as whole genome or whole exome sequencing (WGS/WES) and RNAseq(uencing)) allows us to deeply, uniformly and reproducibly genetically characterize AML cells. This will further improve risk-group stratification for patients across our consortium.
- WGS and transcriptome (RNAseq) analysis of AML cells provides information on gene polymorphisms/abberations and gene activity, respectively. This information can be used to incorporate tailored and targeted treatment. This will be introduced in the CHIP-AML22 protocol.
- Introduction of advanced treatment-response evaluation by MRD (minimal residual disease) was one of the factors that contributed to the success of the AML12 study. MRD measures the frequency of residual leukemic cells at certain time-points during treatment. MRD is currently measured using flow cytometry but can also be performed by molecular/genetic methods. Further implementation and evaluation of both of these methods both during and following treatment, across the whole consortium, is the third overall objective in this application.
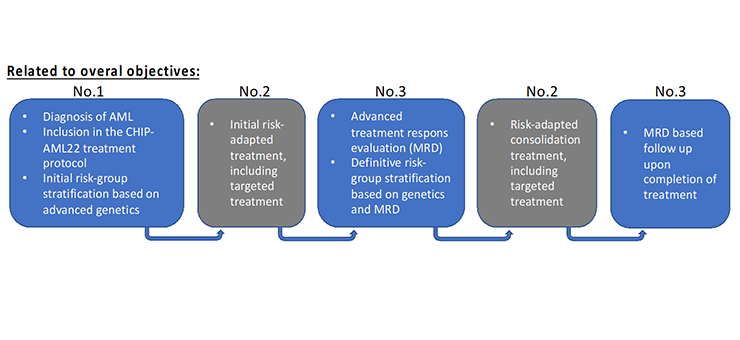
The figure below displays the trajectory of an AML-patient, from initial diagnosis, to treatment, to treatment evaluation and follow-up. Indicated for each phase of the trajectory is the relation to the above mentioned overall objects (nr 1-3). In the annexes to this application, the whole CHIP-AML22 treatment protocol is attached, including all diagnostic and treatment details.
Related to overall objectives:
Step 1 (Related to objective No.1)
- Diagnosis of AML
- Inclusion in the CHIP-AM22 treatment protocol
- Initial risk-group stratification based on advanced search
Step 2 (Related to objective No.2)
- Initial risk-adapted treatment including targeted treatment
Step 3 (Related to objective No.3)
- Advanced treatment respons evaluation (MRD)
- Definitive risk-group stratification based om genetics and MRD
Step 4 (Related to No.2)
- Risk-adapted consolidation treatment including targeted treatment
Step 5 (Related to No.3)
- MRD based follow up upon completion of treatment