Allergies in people with type 1 diabetes are tracked with world-leading investigations
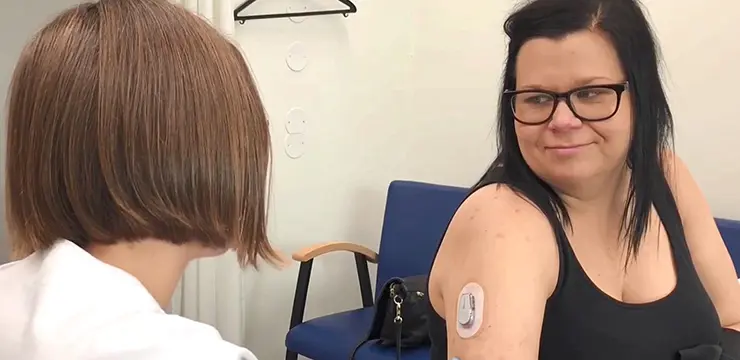
“Patients who become allergic get itchy skin reactions at the site of their application after having used the medical device for a long time”, says Josefin Ulriksdotter, physician who conducts research on the subject at the Department of Occupational and Environmental Dermatology at Skåne University Hospital.
Josefin Ulriksdotter.
The advantage of wearing glucose sensors and insulin pumps directly attached to the skin is that glucose levels can be measured continuously and insulin can be given directly via the pump, which simplifies life for people with type 1 diabetes. A number of users however get side-effects with skin reactions that may vary in intensity from itch to oozing dermatitis.
At the Department of Occupational and Environmental Dermatology, advanced contact allergy investigations are carried out to learn which substances patients may be allergic to. The department is world-unique thanks to a well-equipped laboratory.
“We believe that there are a number of unrecorded cases. There is a big debate on social media where many with severe skin reactions try to get tips from each other. Not many people know that our department exist and what we can offer”, Josefin Ulriksdotter says.
Martin Mowitz, researcher at the department, was the first in the world to identify the substance Isobornyl acrylate, which has caused allergies in many diabetic patients. The discovery had a major international impact and has also led to a large manufacturer of glucose sensors removing the substance from their products.
Martin Mowitz.
Medical devices do not need to be content-declared, but thanks to advanced equipment, the laboratory can examine which substances a product contains and identify allergens that are tested on patients. These are unusual substances that ordinary dermatology clinics do not have access to.
“The problem is that the substances in glucose sensors and insulin pumps are constantly changing, so we must constantly examine what the medical devices contain. In recent months, we have found new allergens in products we previously investigated”, Martin Mowitz says.
Getting an established allergy also has consequences for the patient.
“Despite severe allergic contact dermatitis, patients often want to continue using the products. But a contact allergy is lifelong and those who have become allergic can react even to small doses of the substance. If we are lucky, we can recommend the patient another product that does not contain that exact substance the patient is allergic to. But since many products contain the same or similar substances, sometimes the only alternative may be to go back to traditional diabetes treatments”, Josefin Ulriksdotter says.

